see also: Swagger/OpenAPI definition
ORSCF-VisitData Schema Specification
| Info | |
|---|---|
| author: | ORSCF ("Open Research Study Communication Formats") / T.Korn |
| license: | Apache-2 |
| version: | 1.5.0 |
| timestamp: | 2021-09-11 12:41 |
Contents
- . StudyEvent
- . StudyExecutionScope
- . Visit
- ........\ DataRecording
- ........\ DrugApplyment
- ........\ Treatment
Model:
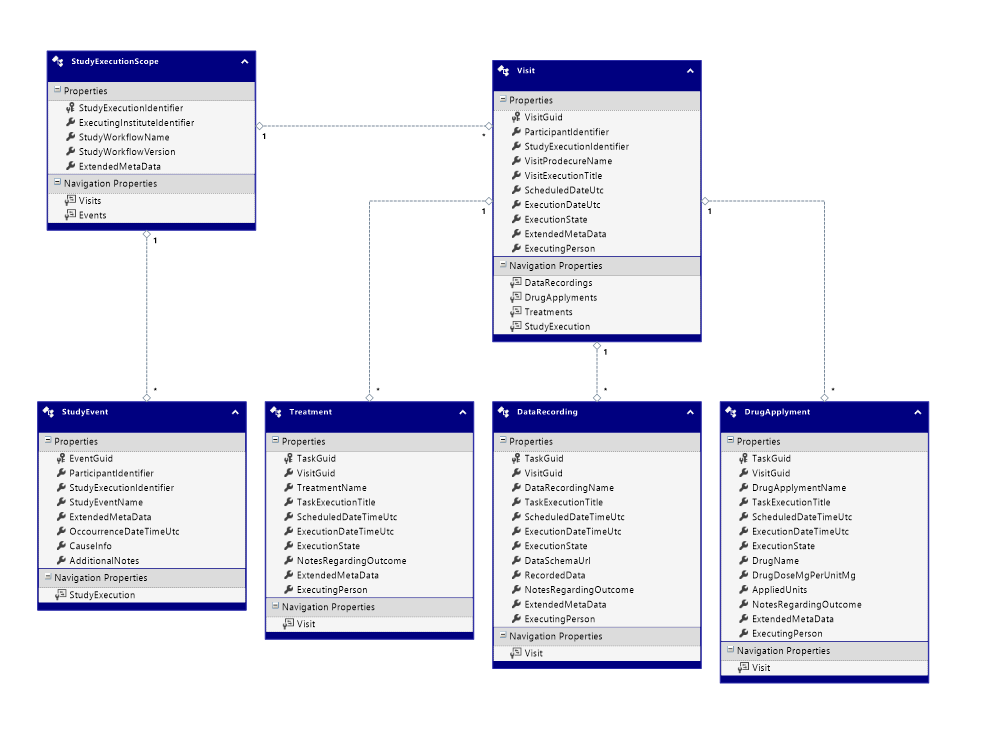
StudyEvent
Fields
| Name | Type | Required | Fix |
|---|---|---|---|
| EventGuid (PK) | guid | YES | no |
| ParticipantIdentifier | string | YES | no |
| StudyExecutionIdentifier (FK) | guid | YES | no |
| StudyEventName | string | YES | no |
| ExtendedMetaData | string | no | no |
| OccourrenceDateTimeUtc | datetime | YES | no |
| CauseInfo | string | YES | no |
| AdditionalNotes | string | no | no |
Unique Keys
- EventGuid (primary)
StudyEvent.EventGuid (Field)
a global unique id of a concrete study-event occurrence which is usually originated at the primary CRF or study management system ('SMS')
- this field represents the identity (PK) of the record
StudyEvent.ParticipantIdentifier (Field)
identity of the patient which can be a randomization or screening number (the exact semantic is defined per study)
StudyEvent.StudyExecutionIdentifier (Field)
a global unique id of a concrete study execution (dedicated to a concrete institute) which is usually originated at the primary CRF or study management system ('SMS')
- this field is used as foreign key to address the related 'StudyExecution'
StudyEvent.StudyEventName (Field)
unique invariant name of the event as defined in the 'StudyWorkflowDefinition' (originated from the sponsor)
StudyEvent.ExtendedMetaData (Field)
optional structure (in JSON-format) containing additional metadata regarding this record, which can be used by 'StudyExecutionSystems' to extend the schema
- this field is optional, so that 'null' values are supported
StudyEvent.AdditionalNotes (Field)
additional notes (supplied by the execution person)
- this field is optional, so that 'null' values are supported
Relations
| Navigation-Name | Role | Target-Type | Target-Multiplicity |
|---|---|---|---|
| StudyExecution | Lookup | StudyExecutionScope | 0/1 (optional) |
StudyExecution (lookup from this StudyEvent)
Target Type: StudyExecutionScope Addressed by: StudyExecutionIdentifier.
StudyExecutionScope
Fields
| Name | Type | Required | Fix |
|---|---|---|---|
| StudyExecutionIdentifier (PK) | guid | YES | YES |
| ExecutingInstituteIdentifier | string | YES | YES |
| StudyWorkflowName | string (100) | YES | YES |
| StudyWorkflowVersion | string (20) | YES | YES |
| ExtendedMetaData | string | no | no |
Unique Keys
- StudyExecutionIdentifier (primary)
StudyExecutionScope.StudyExecutionIdentifier (Field)
a global unique id of a concrete study execution (dedicated to a concrete institute) which is usually originated at the primary CRF or study management system ('SMS')
- this field represents the identity (PK) of the record
- after the record has been created, the value of this field must not be changed any more!
StudyExecutionScope.ExecutingInstituteIdentifier (Field)
the institute which is executing the study (this should be an invariant technical representation of the company name or a guid)
- after the record has been created, the value of this field must not be changed any more!
StudyExecutionScope.StudyWorkflowName (Field)
the official invariant name of the study as given by the sponsor
- the maximum length of the content within this field is 100 characters.
- after the record has been created, the value of this field must not be changed any more!
StudyExecutionScope.StudyWorkflowVersion (Field)
version of the workflow
- the maximum length of the content within this field is 20 characters.
- after the record has been created, the value of this field must not be changed any more!
StudyExecutionScope.ExtendedMetaData (Field)
optional structure (in JSON-format) containing additional metadata regarding this record, which can be used by 'StudyExecutionSystems' to extend the schema
- this field is optional, so that 'null' values are supported
Relations
| Navigation-Name | Role | Target-Type | Target-Multiplicity |
|---|---|---|---|
| Events | Referers | StudyEvent | * (multiple) |
| Visits | Referers | Visit | * (multiple) |
Events (refering to this StudyExecutionScope)
Target: StudyEvent
Visits (refering to this StudyExecutionScope)
Target: Visit
Visit
Fields
| Name | Type | Required | Fix |
|---|---|---|---|
| VisitGuid (PK) | guid | YES | YES |
| ParticipantIdentifier | string (50) | YES | YES |
| StudyExecutionIdentifier (FK) | guid | YES | no |
| VisitProdecureName | string | YES | no |
| VisitExecutionTitle | string | YES | no |
| ScheduledDateUtc | datetime | no | no |
| ExecutionDateUtc | datetime | no | no |
| ExecutionState | int32 | YES | no |
| ExtendedMetaData | string | no | no |
| ExecutingPerson | string | no | no |
Unique Keys
- VisitGuid (primary)
- ParticipantIdentifier + StudyExecutionIdentifier + VisitExecutionTitle
Visit.VisitGuid (Field)
a global unique id of a concrete study-visit execution which is usually originated at the primary CRF or study management system ('SMS')
- this field represents the identity (PK) of the record
- after the record has been created, the value of this field must not be changed any more!
Visit.ParticipantIdentifier (Field)
identity of the patient which can be a randomization or screening number (the exact semantic is defined per study)
- the maximum length of the content within this field is 50 characters.
- after the record has been created, the value of this field must not be changed any more!
Visit.StudyExecutionIdentifier (Field)
a global unique id of a concrete study execution (dedicated to a concrete institute) which is usually originated at the primary CRF or study management system ('SMS')
- this field is used as foreign key to address the related 'StudyExecution'
Visit.VisitProdecureName (Field)
unique invariant name of the visit-procedure as defined in the 'StudyWorkflowDefinition' (originated from the sponsor)
Visit.VisitExecutionTitle (Field)
unique title of the visit execution as defined in the 'StudyWorkflowDefinition' (originated from the sponsor)
Visit.ScheduledDateUtc (Field)
the estimated date when the visit is scheduled for execution
- this field is optional, so that 'null' values are supported
Visit.ExecutionDateUtc (Field)
the real date, when the visits was executed
- this field is optional, so that 'null' values are supported
Visit.ExecutionState (Field)
0=Unscheduled / 1=Sheduled / 2=Executed / 3=AbortDuringExecution / 4=Skipped / 5=Removed
Visit.ExtendedMetaData (Field)
optional structure (in JSON-format) containing additional metadata regarding this record, which can be used by 'StudyExecutionSystems' to extend the schema
- this field is optional, so that 'null' values are supported
Visit.ExecutingPerson (Field)
- this field is optional, so that 'null' values are supported
Relations
| Navigation-Name | Role | Target-Type | Target-Multiplicity |
|---|---|---|---|
| DataRecordings | Childs | DataRecording | * (multiple) |
| DrugApplyments | Childs | DrugApplyment | * (multiple) |
| StudyExecution | Lookup | StudyExecutionScope | 0/1 (optional) |
| Treatments | Childs | Treatment | * (multiple) |
DataRecordings (childs of this Visit)
Target: DataRecording
all the data which has been captured for this visit
DrugApplyments (childs of this Visit)
Target: DrugApplyment
all the drug applyments which have been executed for this visit
StudyExecution (lookup from this Visit)
Target Type: StudyExecutionScope Addressed by: StudyExecutionIdentifier.
Treatments (childs of this Visit)
Target: Treatment
all the treatments which have been executed for this visit
DataRecording
Fields
| Name | Type | Required | Fix |
|---|---|---|---|
| TaskGuid (PK) | guid | YES | YES |
| VisitGuid (FK) | guid | YES | no |
| DataRecordingName | string | YES | no |
| TaskExecutionTitle | string | YES | no |
| ScheduledDateTimeUtc | datetime | no | no |
| ExecutionDateTimeUtc | datetime | no | no |
| ExecutionState | int32 | YES | no |
| DataSchemaUrl | string | YES | no |
| RecordedData | string | YES | no |
| NotesRegardingOutcome | string | no | no |
| ExtendedMetaData | string | YES | no |
| ExecutingPerson | string | no | no |
Unique Keys
- TaskGuid (primary)
DataRecording.TaskGuid (Field)
a global unique id of a concrete study-task execution which is usually originated at the primary CRF or study management system ('SMS')
- this field represents the identity (PK) of the record
- after the record has been created, the value of this field must not be changed any more!
DataRecording.VisitGuid (Field)
the guid of the visit in which this task was executed
- this field is used as foreign key to address the related 'Visit'
DataRecording.DataRecordingName (Field)
unique invariant name of ths task-procedure as defined in the 'StudyWorkflowDefinition' (originated from the sponsor)
DataRecording.TaskExecutionTitle (Field)
title of the task execution as defined in the 'StudyWorkflowDefinition' (originated from the sponsor)
DataRecording.ScheduledDateTimeUtc (Field)
the estimated date when the visit is scheduled
- this field is optional, so that 'null' values are supported
DataRecording.ExecutionDateTimeUtc (Field)
the real time, when the data was recorded
- this field is optional, so that 'null' values are supported
DataRecording.ExecutionState (Field)
0=Unscheduled / 1=Sheduled / 2=Executed / 3=AbortDuringExecution / 4=Skipped / 5=Removed
DataRecording.DataSchemaUrl (Field)
schema-url of the data which were stored inside of the 'RecordedData' field
DataRecording.RecordedData (Field)
RAW data, in the schema as defined at the 'DataSchemaUrl'
DataRecording.NotesRegardingOutcome (Field)
additional notes regarding this dedcated execution (supplied by the execution person)
- this field is optional, so that 'null' values are supported
DataRecording.ExtendedMetaData (Field)
optional structure (in JSON-format) containing additional metadata regarding this record, which can be used by 'StudyExecutionSystems' to extend the schema
DataRecording.ExecutingPerson (Field)
- this field is optional, so that 'null' values are supported
Relations
| Navigation-Name | Role | Target-Type | Target-Multiplicity |
|---|---|---|---|
| Visit | Parent | Visit | 0/1 (optional) |
Visit (parent of this DataRecording)
Target Type: Visit Addressed by: VisitGuid.
DrugApplyment
Fields
| Name | Type | Required | Fix |
|---|---|---|---|
| TaskGuid (PK) | guid | YES | YES |
| VisitGuid (FK) | guid | YES | no |
| DrugApplymentName | string | YES | no |
| TaskExecutionTitle | string | YES | no |
| ScheduledDateTimeUtc | datetime | no | no |
| ExecutionDateTimeUtc | datetime | no | no |
| ExecutionState | int32 | YES | no |
| DrugName | string | YES | no |
| DrugDoseMgPerUnitMg | decimal | YES | no |
| AppliedUnits | decimal | YES | no |
| NotesRegardingOutcome | string | no | no |
| ExtendedMetaData | string | YES | no |
| ExecutingPerson | string | no | no |
Unique Keys
- TaskGuid (primary)
DrugApplyment.TaskGuid (Field)
a global unique id of a concrete study-task execution which is usually originated at the primary CRF or study management system ('SMS')
- this field represents the identity (PK) of the record
- after the record has been created, the value of this field must not be changed any more!
DrugApplyment.VisitGuid (Field)
the guid of the visit in which this task was executed
- this field is used as foreign key to address the related 'Visit'
DrugApplyment.DrugApplymentName (Field)
unique invariant name of the study itself as defined in the 'StudyWorkflowDefinition' (originated from the sponsor)
DrugApplyment.TaskExecutionTitle (Field)
title of the task execution as defined in the 'StudyWorkflowDefinition' (originated from the sponsor)
DrugApplyment.ScheduledDateTimeUtc (Field)
the estimated time when the drug applyment is scheduled
- this field is optional, so that 'null' values are supported
DrugApplyment.ExecutionDateTimeUtc (Field)
the real date, when the visits was executed
- this field is optional, so that 'null' values are supported
DrugApplyment.ExecutionState (Field)
0=Unscheduled / 1=Sheduled / 2=Executed / 3=AbortDuringExecution / 4=Skipped / 5=Removed
DrugApplyment.DrugName (Field)
name of the drug
DrugApplyment.DrugDoseMgPerUnitMg (Field)
dose (mg) which is inside of one unit
DrugApplyment.AppliedUnits (Field)
amount of applied units
DrugApplyment.NotesRegardingOutcome (Field)
additional notes regarding this dedcated execution (supplied by the execution person)
- this field is optional, so that 'null' values are supported
DrugApplyment.ExtendedMetaData (Field)
optional structure (in JSON-format) containing additional metadata regarding this record, which can be used by 'StudyExecutionSystems' to extend the schema
DrugApplyment.ExecutingPerson (Field)
- this field is optional, so that 'null' values are supported
Relations
| Navigation-Name | Role | Target-Type | Target-Multiplicity |
|---|---|---|---|
| Visit | Parent | Visit | 0/1 (optional) |
Visit (parent of this DrugApplyment)
Target Type: Visit Addressed by: VisitGuid.
Treatment
Fields
| Name | Type | Required | Fix |
|---|---|---|---|
| TaskGuid (PK) | guid | YES | YES |
| VisitGuid (FK) | guid | YES | no |
| TreatmentName | string | YES | no |
| TaskExecutionTitle | string | YES | no |
| ScheduledDateTimeUtc | datetime | no | no |
| ExecutionDateTimeUtc | datetime | no | no |
| ExecutionState | int32 | YES | no |
| NotesRegardingOutcome | string | no | no |
| ExtendedMetaData | string | YES | no |
| ExecutingPerson | string | no | no |
Unique Keys
- TaskGuid (primary)
Treatment.TaskGuid (Field)
a global unique id of a concrete study-task execution which is usually originated at the primary CRF or study management system ('SMS')
- this field represents the identity (PK) of the record
- after the record has been created, the value of this field must not be changed any more!
Treatment.VisitGuid (Field)
the guid of the visit in which this task was executed
- this field is used as foreign key to address the related 'Visit'
Treatment.TreatmentName (Field)
unique invariant name of ths task-procedure as defined in the 'StudyWorkflowDefinition' (originated from the sponsor)
Treatment.TaskExecutionTitle (Field)
title of the task execution as defined in the 'StudyWorkflowDefinition' (originated from the sponsor)
Treatment.ScheduledDateTimeUtc (Field)
the estimated time when the treatment is scheduled
- this field is optional, so that 'null' values are supported
Treatment.ExecutionDateTimeUtc (Field)
the real time, when the treatment was executed
- this field is optional, so that 'null' values are supported
Treatment.ExecutionState (Field)
0=Unscheduled / 1=Sheduled / 2=Executed / 3=AbortDuringExecution / 4=Skipped / 5=Removed
Treatment.NotesRegardingOutcome (Field)
additional notes regarding this dedcated execution (supplied by the execution person)
- this field is optional, so that 'null' values are supported
Treatment.ExtendedMetaData (Field)
optional structure of additional metadata regarding this record in JSON-format, which can be used by study execution systems to extend the schema
Treatment.ExecutingPerson (Field)
- this field is optional, so that 'null' values are supported
Relations
| Navigation-Name | Role | Target-Type | Target-Multiplicity |
|---|---|---|---|
| Visit | Parent | Visit | 0/1 (optional) |