ORSCF-StudyManagement Schema Specification
| Info | |
|---|---|
| author: | ORSCF ("Open Research Study Communication Formats") / T.Korn |
| license: | Apache-2 |
| version: | 1.6.0 |
| timestamp: | 2021-11-28 00:00 |
Contents
- . Institute
- ........\ InstituteRelatedSystemAssignment
- ........\ SystemConnection
- ........\ SystemEndpoint
- . InvolvedPerson
- . ResearchStudy
- ........\ InvolvementRole
- ........\ Site
- ................\ SiteRelatedSystemAssignment
- ........\ StudyRelatedSystemAssignment
Model:
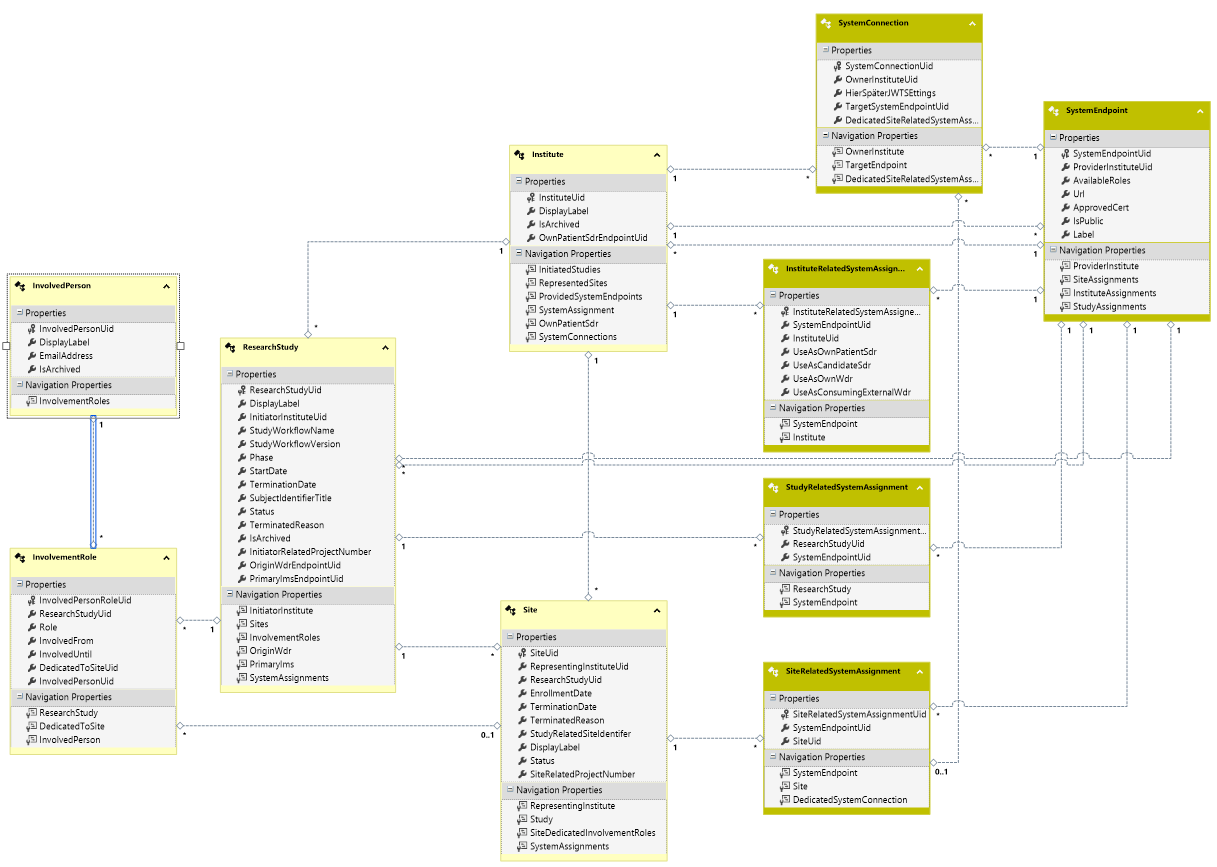
Institute
Fields
| Name | Type | Required | Fix |
|---|---|---|---|
| InstituteUid (PK) | guid | YES | no |
| DisplayLabel | string (100) | YES | no |
| IsArchived | boolean | YES | no |
Unique Keys
- InstituteUid (primary)
Institute.InstituteUid (Field)
An Universally Unique Identifier which can be generated by any origin system and is used to address this ORSCF conform data record in decentralized environments. Note that this Identity must not be changed any more!
- this field represents the identity (PK) of the record
Institute.DisplayLabel (Field)
An DisplayLabel which is dedicated for the usage within the frontend of study managent software. Note that this short name representation hat the caracter of an internal shortcurt and could be ambiguous. The usage for legal-relevant/official communication or documents is not recommended, as well as the usage for technical identification of this record.
- the maximum length of the content within this field is 100 characters.
Relations
| Navigation-Name | Role | Target-Type | Target-Multiplicity |
|---|---|---|---|
| InitiatedStudies | Referers | ResearchStudy | * (multiple) |
| RepresentedSites | Referers | Site | * (multiple) |
| ProvidedSystemEndpoints | Childs | SystemEndpoint | * (multiple) |
| SystemAssignment | Childs | InstituteRelatedSystemAssignment | * (multiple) |
| SystemConnections | Childs | SystemConnection | * (multiple) |
InitiatedStudies (refering to this Institute)
Target: ResearchStudy
RepresentedSites (refering to this Institute)
Target: Site
ProvidedSystemEndpoints (childs of this Institute)
Target: SystemEndpoint
SystemAssignment (childs of this Institute)
Target: InstituteRelatedSystemAssignment
SystemConnections (childs of this Institute)
Target: SystemConnection
InstituteRelatedSystemAssignment
Fields
| Name | Type | Required | Fix |
|---|---|---|---|
| InstituteRelatedSystemAssignemntUid (PK) | guid | YES | no |
| SystemEndpointUid (FK) | guid | YES | no |
| InstituteUid (FK) | guid | YES | no |
| UseAsOwnPatientSdr | string | YES | no |
| UseAsCandidateSdr | string | YES | no |
| UseAsOwnWdr | string | YES | no |
| UseAsConsumingExternalWdr | string | YES | no |
| CustomRoles | string | YES | no |
Unique Keys
- InstituteRelatedSystemAssignemntUid (primary)
InstituteRelatedSystemAssignment.InstituteRelatedSystemAssignemntUid (Field)
- this field represents the identity (PK) of the record
InstituteRelatedSystemAssignment.SystemEndpointUid (Field)
- this field is used as foreign key to address the related 'SystemEndpoint'
InstituteRelatedSystemAssignment.InstituteUid (Field)
- this field is used as foreign key to address the related 'Institute'
InstituteRelatedSystemAssignment.CustomRoles (Field)
semicolon separated list of custom role-names
Relations
| Navigation-Name | Role | Target-Type | Target-Multiplicity |
|---|---|---|---|
| Institute | Parent | Institute | 0/1 (optional) |
| SystemEndpoint | Lookup | SystemEndpoint | 0/1 (optional) |
Institute (parent of this InstituteRelatedSystemAssignment)
Target Type: Institute Addressed by: InstituteUid.
SystemEndpoint (lookup from this InstituteRelatedSystemAssignment)
Target Type: SystemEndpoint Addressed by: SystemEndpointUid.
SystemConnection
Fields
| Name | Type | Required | Fix |
|---|---|---|---|
| SystemConnectionUid (PK) | guid | YES | no |
| OwnerInstituteUid (FK) | guid | YES | no |
| HierSpšterJWTSEttings | string | YES | no |
| TargetSystemEndpointUid (FK) | guid | YES | no |
| DedicatedSiteRelatedSystemAssignmentUid (FK) | guid | no | no |
Unique Keys
- SystemConnectionUid (primary)
SystemConnection.SystemConnectionUid (Field)
- this field represents the identity (PK) of the record
SystemConnection.OwnerInstituteUid (Field)
- this field is used as foreign key to address the related 'OwnerInstitute'
SystemConnection.TargetSystemEndpointUid (Field)
- this field is used as foreign key to address the related 'TargetEndpoint'
SystemConnection.DedicatedSiteRelatedSystemAssignmentUid (Field)
- this field is optional, so that 'null' values are supported
- this field is used as foreign key to address the related 'DedicatedSiteRelatedSystemAssignment'
Relations
| Navigation-Name | Role | Target-Type | Target-Multiplicity |
|---|---|---|---|
| OwnerInstitute | Parent | Institute | 0/1 (optional) |
| DedicatedSiteRelatedSystemAssignment | Lookup | SiteRelatedSystemAssignment | 1 (required) |
| TargetEndpoint | Lookup | SystemEndpoint | 0/1 (optional) |
OwnerInstitute (parent of this SystemConnection)
Target Type: Institute Addressed by: OwnerInstituteUid.
DedicatedSiteRelatedSystemAssignment (lookup from this SystemConnection)
Target Type: SiteRelatedSystemAssignment Addressed by: DedicatedSiteRelatedSystemAssignmentUid.
TargetEndpoint (lookup from this SystemConnection)
Target Type: SystemEndpoint Addressed by: TargetSystemEndpointUid.
SystemEndpoint
Fields
| Name | Type | Required | Fix |
|---|---|---|---|
| SystemEndpointUid (PK) | guid | YES | no |
| ProviderInstituteUid (FK) | guid | YES | no |
| AvailableRoles | string | YES | no |
| Url | string | YES | no |
| ApprovedCert | string | YES | no |
| IsPublic | string | YES | no |
| Label | string | YES | no |
Unique Keys
- SystemEndpointUid (primary)
SystemEndpoint.SystemEndpointUid (Field)
- this field represents the identity (PK) of the record
SystemEndpoint.ProviderInstituteUid (Field)
- this field is used as foreign key to address the related 'ProviderInstitute'
Relations
| Navigation-Name | Role | Target-Type | Target-Multiplicity |
|---|---|---|---|
| ProviderInstitute | Parent | Institute | 0/1 (optional) |
| InstituteAssignments | Referers | InstituteRelatedSystemAssignment | * (multiple) |
| SiteAssignments | Referers | SiteRelatedSystemAssignment | * (multiple) |
| StudyAssignments | Referers | StudyRelatedSystemAssignment | * (multiple) |
ProviderInstitute (parent of this SystemEndpoint)
Target Type: Institute Addressed by: ProviderInstituteUid.
InstituteAssignments (refering to this SystemEndpoint)
Target: InstituteRelatedSystemAssignment
SiteAssignments (refering to this SystemEndpoint)
Target: SiteRelatedSystemAssignment
StudyAssignments (refering to this SystemEndpoint)
Target: StudyRelatedSystemAssignment
InvolvedPerson
Fields
| Name | Type | Required | Fix |
|---|---|---|---|
| InvolvedPersonUid (PK) | guid | YES | no |
| DisplayLabel | string | no | no |
| EmailAddress | guid | no | no |
| IsArchived | boolean | YES | no |
Unique Keys
- InvolvedPersonUid (primary)
InvolvedPerson.InvolvedPersonUid (Field)
An Universally Unique Identifier which can be generated by any origin system and is used to address this ORSCF conform data record in decentralized environments. Note that this Identity must not be changed any more!
- this field represents the identity (PK) of the record
InvolvedPerson.DisplayLabel (Field)
- this field is optional, so that 'null' values are supported
InvolvedPerson.EmailAddress (Field)
- this field is optional, so that 'null' values are supported
Relations
| Navigation-Name | Role | Target-Type | Target-Multiplicity |
|---|---|---|---|
| InvolvementRoles | Referers | InvolvementRole | * (multiple) |
InvolvementRoles (refering to this InvolvedPerson)
Target: InvolvementRole
ResearchStudy
entity, which relates to HL7.ResearchStudy
Fields
| Name | Type | Required | Fix |
|---|---|---|---|
| ResearchStudyUid (PK) | guid | YES | no |
| DisplayLabel | string (100) | YES | no |
| InitiatorInstituteUid (FK) | guid | YES | no |
| StudyWorkflowName | string | YES | no |
| StudyWorkflowVersion | string | YES | no |
| Phase | string | no | no |
| StartDate | datetime | no | no |
| TerminationDate | datetime | no | no |
| SubjectIdentifierTitle | string | YES | no |
| Status | string | YES | no |
| TerminatedReason | string | no | no |
| IsArchived | boolean | YES | no |
| InitiatorRelatedProjectNumber | string | no | no |
| OriginWdrEndpointUid (FK) | guid | no | no |
Unique Keys
- ResearchStudyUid (primary)
ResearchStudy.ResearchStudyUid (Field)
An Universally Unique Identifier which can be generated by any origin system and is used to address this ORSCF conform data record in decentralized environments. Note that this Identity must not be changed any more!
- this field represents the identity (PK) of the record
ResearchStudy.DisplayLabel (Field)
An DisplayLabel which is dedicated for the usage within the frontend of study managent software. Note that this short name representation hat the caracter of an internal shortcurt and could be ambiguous. The usage for legal-relevant/official communication or documents is not recommended, as well as the usage for technical identification of this record.
- the maximum length of the content within this field is 100 characters.
ResearchStudy.InitiatorInstituteUid (Field)
- this field is used as foreign key to address the related 'InitiatorInstitute'
ResearchStudy.Phase (Field)
AS DECLARED BY HL7.ResearchStudyPhase: n-a | early-phase-1 | phase-1 | phase-1-phase-2 | phase-2 | phase-2-phase-3 | phase-3 | phase-4
- this field is optional, so that 'null' values are supported
ResearchStudy.StartDate (Field)
a estimated date in future is possible
- this field is optional, so that 'null' values are supported
ResearchStudy.TerminationDate (Field)
a estimated date in future is possible
- this field is optional, so that 'null' values are supported
ResearchStudy.SubjectIdentifierTitle (Field)
A title which informs about the sematic of the SubjectIdentifer (which concrete value is used): "Randomization-Number", "Screening-Number", ...
ResearchStudy.Status (Field)
AS DECLARED BY HL7.ResearchStudyStatus: active | administratively-completed | approved | closed-to-accrual | closed-to-accrual-and-intervention | completed | disapproved | in-review | temporarily-closed-to-accrual | temporarily-closed-to-accrual-and-intervention | withdrawn
ResearchStudy.TerminatedReason (Field)
- this field is optional, so that 'null' values are supported
ResearchStudy.InitiatorRelatedProjectNumber (Field)
- this field is optional, so that 'null' values are supported
ResearchStudy.OriginWdrEndpointUid (Field)
- this field is optional, so that 'null' values are supported
- this field is used as foreign key to address the related 'OriginWdr'
Relations
| Navigation-Name | Role | Target-Type | Target-Multiplicity |
|---|---|---|---|
| InitiatorInstitute | Lookup | Institute | 0/1 (optional) |
| InvolvementRoles | Childs | InvolvementRole | * (multiple) |
| Sites | Childs | Site | * (multiple) |
| OriginWdr | Lookup | SystemEndpoint | 1 (required) |
| SystemAssignments | Childs | StudyRelatedSystemAssignment | * (multiple) |
InitiatorInstitute (lookup from this ResearchStudy)
Target Type: Institute Addressed by: InitiatorInstituteUid.
InvolvementRoles (childs of this ResearchStudy)
Target: InvolvementRole
Sites (childs of this ResearchStudy)
Target: Site
OriginWdr (lookup from this ResearchStudy)
Target Type: SystemEndpoint Addressed by: OriginWdrEndpointUid.
SystemAssignments (childs of this ResearchStudy)
Target: StudyRelatedSystemAssignment
InvolvementRole
Fields
| Name | Type | Required | Fix |
|---|---|---|---|
| InvolvedPersonRoleUid (PK) | guid | YES | no |
| ResearchStudyUid (FK) | guid | YES | no |
| Role | string | no | no |
| InvolvedFrom | datetime | no | no |
| InvolvedUntil | datetime | no | no |
| DedicatedToSiteUid (FK) | guid | no | no |
| InvolvedPersonUid (FK) | guid | YES | no |
Unique Keys
- InvolvedPersonRoleUid (primary)
InvolvementRole.InvolvedPersonRoleUid (Field)
An Universally Unique Identifier which can be generated by any origin system and is used to address this ORSCF conform data record in decentralized environments. Note that this Identity must not be changed any more!
- this field represents the identity (PK) of the record
InvolvementRole.ResearchStudyUid (Field)
- this field is used as foreign key to address the related 'ResearchStudy'
InvolvementRole.Role (Field)
- this field is optional, so that 'null' values are supported
InvolvementRole.InvolvedFrom (Field)
- this field is optional, so that 'null' values are supported
InvolvementRole.InvolvedUntil (Field)
- this field is optional, so that 'null' values are supported
InvolvementRole.DedicatedToSiteUid (Field)
null indicated a site independent global role
- this field is optional, so that 'null' values are supported
- this field is used as foreign key to address the related 'DedicatedToSite'
InvolvementRole.InvolvedPersonUid (Field)
- this field is used as foreign key to address the related 'InvolvedPerson'
Relations
| Navigation-Name | Role | Target-Type | Target-Multiplicity |
|---|---|---|---|
| InvolvedPerson | Lookup | InvolvedPerson | 0/1 (optional) |
| ResearchStudy | Parent | ResearchStudy | 0/1 (optional) |
| DedicatedToSite | Lookup | Site | 1 (required) |
InvolvedPerson (lookup from this InvolvementRole)
Target Type: InvolvedPerson Addressed by: InvolvedPersonUid.
ResearchStudy (parent of this InvolvementRole)
Target Type: ResearchStudy Addressed by: ResearchStudyUid.
DedicatedToSite (lookup from this InvolvementRole)
Target Type: Site Addressed by: DedicatedToSiteUid.
Site
Fields
| Name | Type | Required | Fix |
|---|---|---|---|
| SiteUid (PK) | guid | YES | no |
| RepresentingInstituteUid (FK) | guid | YES | no |
| ResearchStudyUid (FK) | guid | YES | no |
| EnrollmentDate | datetime | no | no |
| TerminationDate | datetime | no | no |
| TerminatedReason | string | no | no |
| StudyRelatedSiteIdentifer | string | YES | no |
| DisplayLabel | string | YES | no |
| Status | string | YES | no |
| SiteRelatedProjectNumber | string | no | no |
Unique Keys
- SiteUid (primary)
Site.SiteUid (Field)
An Universally Unique Identifier which can be generated by any origin system and is used to address this ORSCF conform data record in decentralized environments. Note that this Identity must not be changed any more!
- this field represents the identity (PK) of the record
Site.RepresentingInstituteUid (Field)
Universally Unique Identifier of the institute, which is representing this site
- this field is used as foreign key to address the related 'RepresentingInstitute'
Site.ResearchStudyUid (Field)
Universally Unique Identifier of the related record
- this field is used as foreign key to address the related 'Study'
Site.EnrollmentDate (Field)
a estimated date in future is possible
- this field is optional, so that 'null' values are supported
Site.TerminationDate (Field)
a estimated date in future is possible
- this field is optional, so that 'null' values are supported
Site.TerminatedReason (Field)
- this field is optional, so that 'null' values are supported
Site.StudyRelatedSiteIdentifer (Field)
Offical 'SiteIdentifier' which is unique within the scope of the related study
Site.DisplayLabel (Field)
An DisplayLabel which is dedicated for the usage within the frontend of study managent software. Note that this short name representation hat the caracter of an internal shortcurt and could be ambiguous. The usage for legal-relevant/official communication or documents is not recommended, as well as the usage for technical identification of this record.
Site.Status (Field)
AS DECLARED BY HL7
Site.SiteRelatedProjectNumber (Field)
- this field is optional, so that 'null' values are supported
Relations
| Navigation-Name | Role | Target-Type | Target-Multiplicity |
|---|---|---|---|
| RepresentingInstitute | Lookup | Institute | 0/1 (optional) |
| SiteDedicatedInvolvementRoles | Referers | InvolvementRole | * (multiple) |
| Study | Parent | ResearchStudy | 0/1 (optional) |
| SystemAssignments | Childs | SiteRelatedSystemAssignment | * (multiple) |
RepresentingInstitute (lookup from this Site)
Target Type: Institute Addressed by: RepresentingInstituteUid.
SiteDedicatedInvolvementRoles (refering to this Site)
Target: InvolvementRole
Study (parent of this Site)
Target Type: ResearchStudy Addressed by: ResearchStudyUid.
SystemAssignments (childs of this Site)
Target: SiteRelatedSystemAssignment
SiteRelatedSystemAssignment
Fields
| Name | Type | Required | Fix |
|---|---|---|---|
| SiteRelatedSystemAssignmentUid (PK) | guid | YES | no |
| SystemEndpointUid (FK) | guid | YES | no |
| SiteUid (FK) | guid | YES | no |
| CustomRoles | string | YES | no |
Unique Keys
- SiteRelatedSystemAssignmentUid (primary)
SiteRelatedSystemAssignment.SiteRelatedSystemAssignmentUid (Field)
- this field represents the identity (PK) of the record
SiteRelatedSystemAssignment.SystemEndpointUid (Field)
- this field is used as foreign key to address the related 'SystemEndpoint'
SiteRelatedSystemAssignment.SiteUid (Field)
- this field is used as foreign key to address the related 'Site'
SiteRelatedSystemAssignment.CustomRoles (Field)
semicolon separated list of custom role-names
Relations
| Navigation-Name | Role | Target-Type | Target-Multiplicity |
|---|---|---|---|
| Site | Parent | Site | 0/1 (optional) |
| SystemEndpoint | Lookup | SystemEndpoint | 0/1 (optional) |
| DedicatedSystemConnection | Referers | SystemConnection | * (multiple) |
Site (parent of this SiteRelatedSystemAssignment)
Target Type: Site Addressed by: SiteUid.
SystemEndpoint (lookup from this SiteRelatedSystemAssignment)
Target Type: SystemEndpoint Addressed by: SystemEndpointUid.
DedicatedSystemConnection (refering to this SiteRelatedSystemAssignment)
Target: SystemConnection
StudyRelatedSystemAssignment
Fields
| Name | Type | Required | Fix |
|---|---|---|---|
| StudyRelatedSystemAssignmentUid (PK) | guid | YES | no |
| ResearchStudyUid (FK) | guid | YES | no |
| SystemEndpointUid (FK) | guid | YES | no |
| CustomRoles | string | YES | no |
Unique Keys
- StudyRelatedSystemAssignmentUid (primary)
StudyRelatedSystemAssignment.StudyRelatedSystemAssignmentUid (Field)
- this field represents the identity (PK) of the record
StudyRelatedSystemAssignment.ResearchStudyUid (Field)
- this field is used as foreign key to address the related 'ResearchStudy'
StudyRelatedSystemAssignment.SystemEndpointUid (Field)
- this field is used as foreign key to address the related 'SystemEndpoint'
StudyRelatedSystemAssignment.CustomRoles (Field)
semicolon separated list of custom role-names
Relations
| Navigation-Name | Role | Target-Type | Target-Multiplicity |
|---|---|---|---|
| ResearchStudy | Parent | ResearchStudy | 0/1 (optional) |
| SystemEndpoint | Lookup | SystemEndpoint | 0/1 (optional) |
ResearchStudy (parent of this StudyRelatedSystemAssignment)
Target Type: ResearchStudy Addressed by: ResearchStudyUid.
SystemEndpoint (lookup from this StudyRelatedSystemAssignment)
Target Type: SystemEndpoint Addressed by: SystemEndpointUid.
